Risk Management Program
We operate a risk management program for processing biomaterials. This program manages hazards and other risk factors associated with biomaterials to ensure ‘fitness for purpose’. The program is based on the principles of hazard analysis and critical control points.
Processing Controls
Collagen Solutions ensures compliance with key time and temperature parameters to minimize biodegradation. Our tissue manufacturing facilities are optimally located allowing processing shortly after tissue harvest. Currently customers are served with cross-linking time protocols ranging from 24 to 36 hours’ post-harvest.
BSE-Free Status
Transmissible Spongiform Encephalopathies (TSEs) are diseases caused by infectious agents known as prions. The animal diseases in the group include bovine spongiform encephalopathy (BSE), chronic wasting disease in deer (CWD) and scrapie in sheep. The human disease in this group is Creutzfeldt-Jakob disease (CJD). New Zealand has never had BSE or CWD and has been free of scrapie since 1954. Australia has never had BSE or CWD and has been free of scrapie since 1952.
Biomaterials - Geographic Sourcing
The World Organisation for Animal Health (OIE) sets the criteria for assessment of the status of countries or regions according to their BSE risk. This replaces the GBR system established in 2000 by the European Commission Scientific Steering Committee. The OIE updates the BSErisk status of member countries regularly.
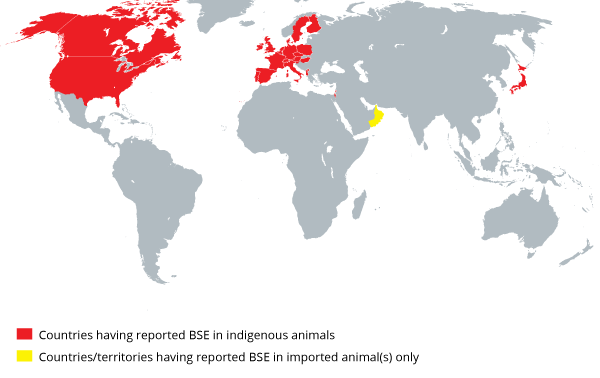
OIE: Geographic distribution of countries that reported at least one BSE confirmed case since 1998.
Biomaterials - Geographic Sourcing
New Zealand and Australia have been recognized by the OIE as a negligible BSE risks countries, this is the highest possible ranking. New Zealand and Australia enjoyed this same stature of ranking under the previous GBR system. The EU indicates that animal tissue to be used in medical devices should be sourced from countries with the lowest possible BSE risk; that is negligible risk countries (EC 2011/C 73/01). The United States is a member country of the OIE and the US Department of Agriculture publishes permitted and authorized source countries (published by the Animal and Plant Health Inspection Service - APHIS). Bovines in New Zealand and Australia are considered low risk herds, as defined in ISO: 22442 Medical Devices utilizing animal tissues and their Derivatives Part 2 Controls on Sourcing, Collection and Handling.




